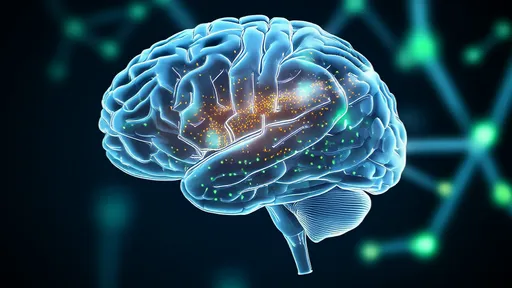
In a landmark advancement for neuroscience, researchers have successfully captured the first three-dimensional in vivo images of dopamine release in the brain using quantum dot-based technology. This breakthrough, emerging from a multi-institutional collaboration, provides an unprecedented view into the real-time dynamics of one of the brain's most crucial neurotransmitters. For decades, scientists have been piecing together the role of dopamine from indirect measurements, but observing its actual ebb and flow in a living organism has remained an elusive goal. The new methodology, detailed in a recent issue of a leading scientific journal, finally lifts the veil, offering a vibrant, dynamic map of neurochemical communication.
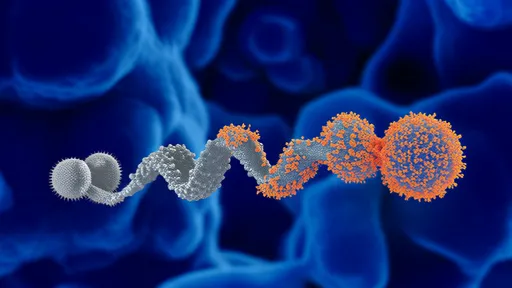
The core of this innovation lies in the application of specially engineered quantum dots—nanoscale semiconductor particles whose fluorescent properties are exquisitely sensitive to their chemical environment. The research team developed a novel probe by tethering these quantum dots to genetically modified dopamine receptors. When a dopamine molecule binds to its receptor, it induces a subtle conformational change. This shift alters the local environment of the quantum dot, causing a measurable and rapid change in its fluorescence intensity. Unlike previous methods that offered a flat, two-dimensional snapshot or required invasive extraction of tissue, this system functions as a highly sensitive, non-destructive biosensor deep within the complex, three-dimensional landscape of the living brain.
Implementing this technology in vivo presented a monumental challenge. The team had to ensure the quantum dot probes were biocompatible, stable, and specific enough to avoid interference from the brain's myriad other signaling molecules. Through meticulous design, they achieved a probe that is both highly selective for dopamine and robust enough to withstand the harsh conditions within brain tissue. Using a customized imaging setup that combines multiphoton microscopy with advanced light-sheet techniques, the researchers can now scan through neural tissue and construct a high-resolution, three-dimensional video of dopamine release events. The resulting data is not just a static picture; it's a movie of neurochemistry in action, showing precisely where, when, and how much dopamine is being released.
The implications of this capability are profound. For the first time, neuroscientists can directly observe the spatiotemporal patterns of dopamine signaling during specific behaviors. Initial experiments have already yielded stunning visuals of dopamine floods in reward pathways when a subject receives an unexpected reward, and the nuanced, wave-like patterns of release during learning tasks. This moves beyond simply knowing that dopamine is involved in a process; it allows scientists to decode the precise language of dopamine communication. This language, it turns out, is far more complex and information-rich than previously imagined, with intricate patterns that likely encode specific details about expectations, errors, and motivations.
This technological leap forward is poised to revolutionize our fundamental understanding of brain function. Dopamine is a key player in a vast array of processes, including motor control, motivation, arousal, and reinforcement learning. Dysfunctions in the dopamine system are central to numerous neurological and psychiatric disorders, such as Parkinson's disease, schizophrenia, addiction, and depression. Prior treatments for these conditions have often been developed with a somewhat blunt understanding of the underlying neurochemical imbalance. This new imaging platform provides a powerful tool to observe these dysfunctions directly in animal models, offering a clear window into what goes wrong at the molecular level in diseased states.
Consequently, the path to developing new therapies appears much brighter. Pharmaceutical researchers can use this technology to observe, in real-time, how experimental drugs affect dopamine release patterns. This allows for a much more nuanced approach to drug design, moving beyond simply boosting or blocking overall dopamine levels towards crafting molecules that can correct or mimic specific, healthy patterns of release. It enables the screening for compounds that can precisely modulate this system, potentially leading to treatments with higher efficacy and fewer side effects. The ability to visualize a drug's mechanism of action in the living brain is a game-changer for neuroscience and neuropharmacology.
Looking ahead, the research team is focused on refining the technology. Their goals include improving the temporal resolution to capture even faster release events and expanding the color palette of their quantum dots. The latter advancement would be a gateway to multiplexed imaging—simultaneously visualizing dopamine alongside other neurotransmitters like glutamate or serotonin. Observing how these different chemical signals interact and coordinate in three-dimensional space would provide an even more holistic and integrated view of brain computation, akin to moving from a solo performance to watching an entire orchestra play.
In conclusion, the successful demonstration of in vivo 3D imaging of dopamine release via quantum dot neuroimaging marks a pivotal moment. It transforms a critical aspect of brain function from an inferred phenomenon into a directly observable one. This doesn't just add a new tool to the neuroscience toolkit; it fundamentally changes the kinds of questions scientists can ask about the brain, behavior, and disease. As this technology continues to evolve, it promises to illuminate the intricate chemical symphony of the brain, note by precise note, bringing us closer than ever to understanding the very essence of our thoughts, actions, and emotions.

By /Aug 25, 2025
By /Aug 25, 2025
By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025
By /Aug 25, 2025

By /Aug 25, 2025
By /Aug 25, 2025

By /Aug 25, 2025
By /Aug 25, 2025
By /Aug 25, 2025
By /Aug 25, 2025
By /Aug 25, 2025

By /Aug 25, 2025
By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025

By /Aug 25, 2025